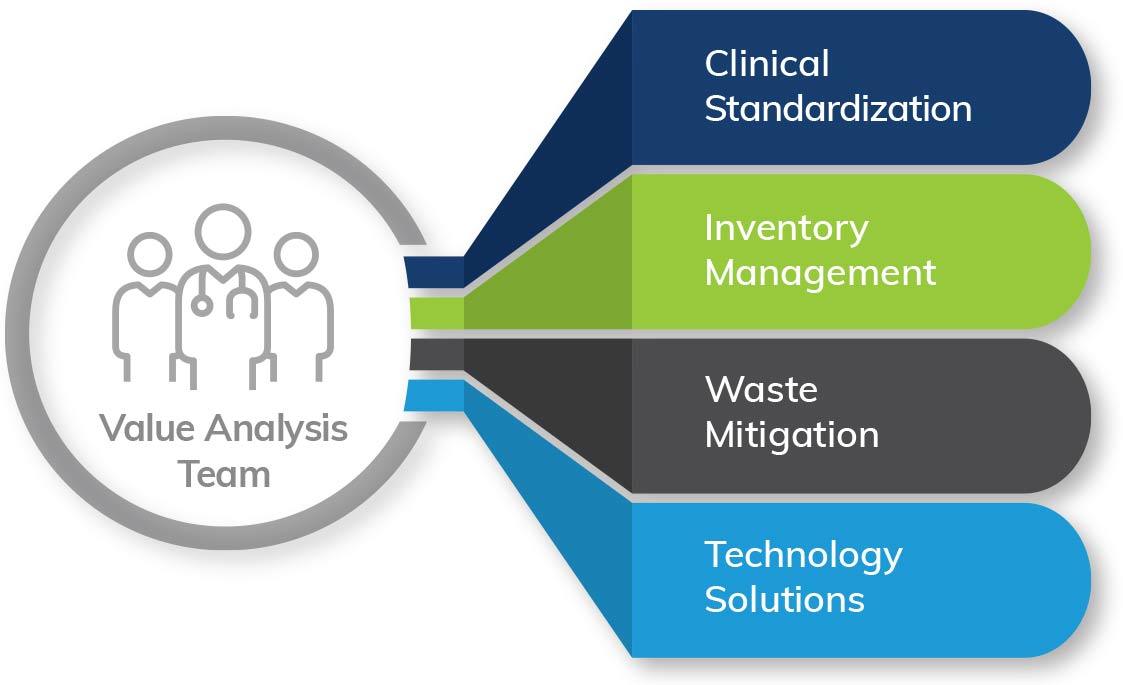
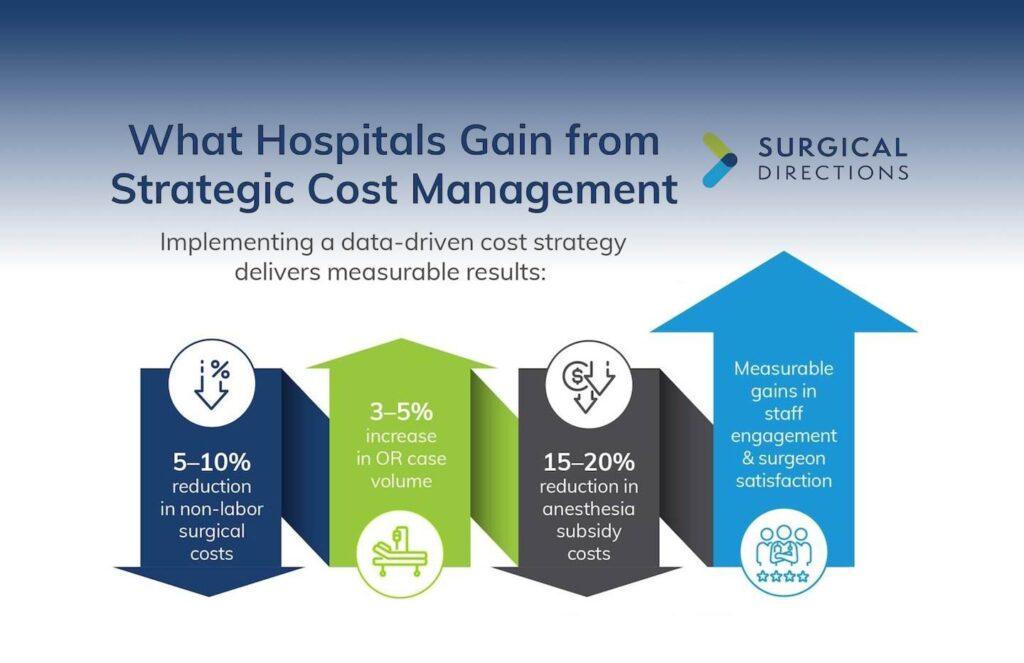
Several factors lead today’s hospitals, health systems, and ASCs to experience negative margins and slow growth. Our consultants understand that optimizing supply chain and materials management can improve operating room efficiency, equipment distribution, and vendor management. These improvements result in:
- Optimized supply chain logistics
- Cost and waste reduction
- Enhanced safety and compliance
- Workforce satisfaction
- Standardization
- Decreased case cancellations and delays