EXECUTIVE SUMMARY
As hospitals strive to improve surgical outcomes, reduce hospital-acquired infections, and achieve compliance levels aligned with national safety standards, one of the most overlooked but critical departments is sterile processing. At the heart of this operation are sterile processing technicians, whose precise, driven work is foundational to patient safety. However, the role remains one of the most difficult to fill in today’s healthcare sector due to its demanding knowledge requirements, physical and mental intensity, and overall lack of recognition within the hospital ecosystem.
This white paper explores the depth of knowledge required to perform the role effectively, the patient care risks that arise when regulatory compliance is compromised, and the urgent investments needed around training, compensation, and career development for this essential workforce sector.
Introduction: The Quiet Core of Surgical Safety
In every hospital operating room (OR), patient safety begins long before the first incision. It starts in the sterile processing department (SPD), a high-stakes environment ensuring every surgical instrument is decontaminated, inspected, assembled, sterilized, stored, and delivered with uncompromising accuracy. Yet, while the OR is visible and revered, the SPD often lingers in the shadows; its professionals rarely acknowledged despite their essential contributions.
Sterile processing is not a routine support service. It is a deeply technical, high-responsibility function that demands rigorous adherence to scientific, regulatory, and procedural standards. Unfortunately, many health systems struggle to fully staff and support their SPD teams. This often leads to surgical delays, safety risks, and dissatisfaction among surgical staff and surgeons. The consequences of these gaps can be serious, resulting in surgical site infections (SSIs), an intraoperative complication, canceled procedures, and regulatory sanctions.
The Knowledge Foundation: What It Really Takes to Become an SPD Technician
Contrary to popular assumptions, sterile processing is not about “washing tools.” It is a knowledge-intensive discipline that integrates life sciences, engineering, infection prevention, logistics, and regulatory compliance.
A Certified SPD Technician Training Includes:
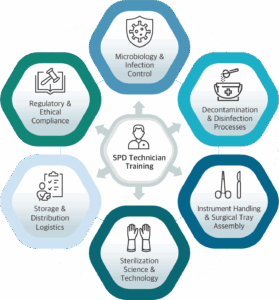
- Microbiology and Infection Control: Technicians must understand the classification of microorganisms, modes of disease transmission, microbial growth conditions, and the mechanisms of biofilm development. This scientific foundation informs the cleaning and sterilization decisions made daily.
- Decontamination and Disinfection Processes: Technicians work with detergents, high-level disinfectants, enzymatic cleaners, and specialized equipment such as ultrasonic cleaners and washer disinfectors. Each chemical must be used according to its instructions for use (IFU), including correct dilution, temperature, contact time and expiration. Moreover, every piece of equipment must be validated, routinely tested, and maintained to ensure consistent performance.
- Instrument Handling and Surgical Tray Assembly: Beyond knowing hundreds of instrument names, technicians must understand how instruments are used in specific procedures and how to inspect them for wear, damage, or malfunction. This includes the configuration of complex trays that must meet both sterilization science and surgical team expectations.
- Sterilization Science and Technology: SPD professionals operate steam, ethylene oxide, hydrogen peroxide plasma, and other sterilizers, applying detailed parameters such as temperature, pressure, exposure time, and humidity. They are also responsible for interpreting biological and chemical indicators, tracking lot numbers, and ensuring documentation is thorough and accurate.
- Storage and Distribution Logistics: Once they are sterile, instruments must be stored under controlled conditions and delivered to the right locations that protects integrity without compromising sterility. This includes inventory management, established rotation systems (First in first out FIFO), and equipment tracking.
- Regulatory and Ethical Compliance: Sterile processing staff must maintain strict compliance with standards and guidelines established by leading regulatory and professional organizations. These include Association of periOperative Registered Nurses, Healthcare Sterile Processing Association, Association for the Advancement of Medical Instrumentation, The Joint Commission, and Centers for Medicare & Medicaid Services. In addition to national and facility-level policies, sterile processing staff are ethically obligated to promptly report non-compliance, unsafe practices, or breaches of protocol. They must follow established procedures for incidents involving biohazard exposure, equipment malfunction, or compromised instrument integrity, ensuring both patient and staff safety remain the highest priority.
This diverse body of knowledge is essential for reducing surgical risks and maintaining patient safety. Yet, despite the technical scope of the role, many SPD technicians are under-supported in both training and compensation.
What’s at Stake: When SPD Fails, Everyone Pays
The sterile processing department is not a luxury; it is a frontline defense. When SPD workflows are compromised, the repercussions ripple across the organization.
According to the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality, SSIs occur in 2-5% of all surgical patients and are frequently linked to contaminated instruments or improper sterilization. These infections increase hospital stays, drive up healthcare costs, and in some cases, result in long-term disability or death for patients.
Moreover, a misassembled tray or unavailable instrument may delay or even cancel a procedure. This disrupts surgeon workflow, increases patient dissatisfaction, and strains the OR schedule. In serious cases, regulatory bodies such as The Joint Commission may cite facilities for lapses in sterile processing, which has the potential of affecting accreditation status.
In addition, the legal and reputational damage caused by a documented sterilization breach can be immense. The financial risk from malpractice claims, fines, and corrective actions far outweigh the cost of adequately staffing, resourcing, and valuing the SPD.
The Staffing Crisis: Why It Is So Hard to Fill These Roles
Despite the significance of the work, sterile processing as a profession faces a chronic workforce shortage. Positions often remain vacant for weeks or months, and turnover is high. Several factors contribute to this ongoing crisis:
- Invisibility and Underappreciation: SPD technicians work behind closed doors, often under high pressure and with little-to-no interaction with or recognition from clinical teams. The lack of visibility can create a sense of isolation and belittlement, especially when compared to the high-profile work of frontline caregivers.
- Low Pay for High Responsibility : While SPD technicians bear enormous responsibility, including the potential to impact patient survival, the pay scale often does not reflect the knowledge, risk, or physical demands of the job. This disparity deters new entrants and contributes to high attrition.
- Limited Career Pathways: Many SPD roles are viewed as “entry level,” with few structured pathways for advancement. This lack of career progression discourages long-term commitment, particularly among younger workers seeking growth opportunities.
- Intense Training Requirements : To be certified, technicians must study complex material in microbiology, sterilization science, anatomy, and more, often while working full time. Without institutional support, many potential candidates struggle to meet these certification demands.
- Physically and Mentally Demanding Work: SPD work is labor intensive, requiring long hours on foot, heavy lifting, exposure to biohazard, and intense focus. In fast-paced surgical environments, the pressure to turn over trays quickly, while also maintaining perfect accuracy, can lead to burnout.
The Way Forward: Elevating the Profession
Despite the significance of the work, sterile processing as a profession faces a chronic workforce shortage. Positions often remain vacant for weeks or months, and turnover is high. Several factors contribute to this ongoing crisis:
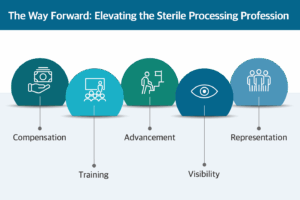
- Improving Compensation: Align wages with the technical complexity and critical nature of the role.
- Investing in Training: Provide structured onboarding programs, certification support, and continuing education.
- Building Career Pathways: Create defined advancement tracks for SPD professionals to grow into leadership, educator, or clinical quality roles.
- Increasing Visibility: Educate surgical staff and hospital leaders about the value and expertise of SPD technicians.
- Integrating SPD into Safety and Governance Structures: Include SPD leaders in infection prevention, OR governance, and quality meetings to ensure their insights shape hospital-wide decisions.
Conclusion: Sterile processing technicians are the silent backbone of surgical care. Their role is not only highly technical but also profoundly consequential. Every instrument that enters a patient’s body has passed through their hands, and every step they take, from cleaning to sterilization, is a safeguard against harm.
As healthcare systems confront growing surgical volumes and increasing regulatory demands, sterile processing must be treated not as a cost center, but as a strategic pillar of safe, high-quality care.



